
What Is A Salt?
Understanding Salt Beyond the Table
Chemistry’s Definition of Salt
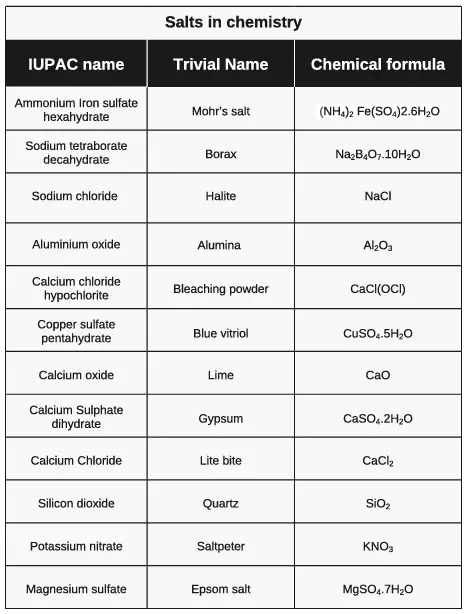
Salt is chemically defined as an ionic compound of positively charged cations and negatively charged anions. It includes far more than the sodium chloride (NaCl) we know as table salt. Instead, salts encompass a diverse range of compounds, both inorganic like chloride or organic like acetate.
What Difference Does Salt Make In Water?
Salts can be categorized based on their behavior in water, with alkali salts producing hydroxide ions and acid salts generating hydrogen ions. Sodium chloride (NaCl), a common salt, dissociates into sodium ions (Na+) and chloride ions (Cl-) in water. This ionization process can increase the electrical conductivity of water, making it more conducive, impacting the electrochemical balance in the environment.
High salt concentrations can also influence the solubility of other compounds and minerals in water, such as enhancing the dissolution of heavy metals and minerals from soils and sediments. This phenomenon can lead to elevated levels of multitudes of contaminants – posing a widespread threat to water quality.

Total Dissolved Solids (TDS)
Measurement of Salt Pollution In Water
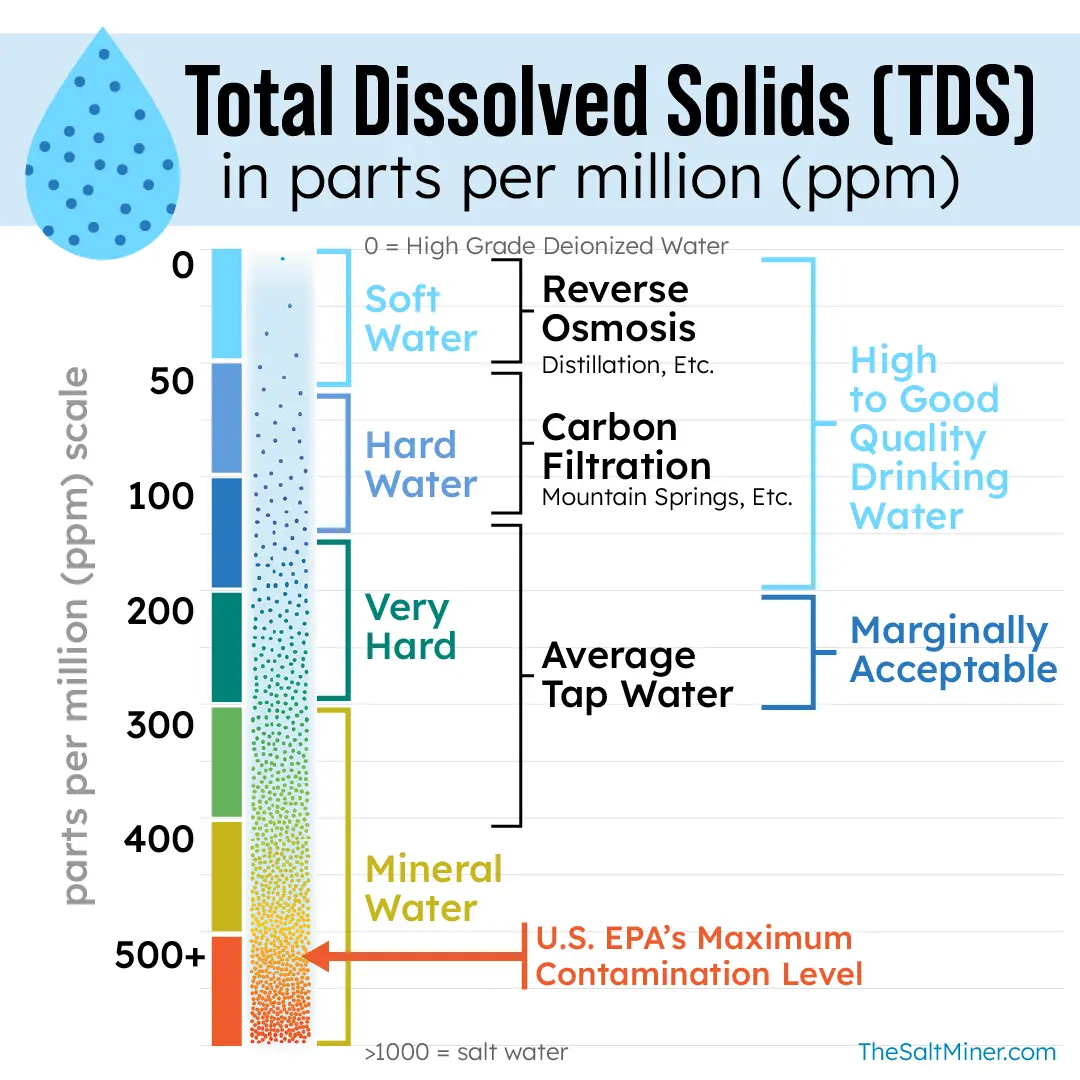
A measurement of Total Dissolved Solids (TDS) gauges water salinity, encapsulating minerals, inorganic salts, and traces of organic matter, and is measured in parts per million (ppm). The Environmental Protection Agency (EPA) sets a minimum standard of 500 ppm TDS for water, which serves not as a “health standard” but a secondary aesthetic guideline.
Monitoring TDS levels is essential in assessing water quality, as elevated TDS values can indicate potential health risks and impact the taste and odor of water. In the context of water treatment, reducing TDS is imperative for achieving a healthier standard of water. High TDS levels can contribute to the formation of scale in pipes and appliances, reducing their efficiency and lifespan. Furthermore, excessive TDS may render water unsuitable for consumption, irrigation, and industrial processes.
